Educational Opportunities
See what’s coming up.


Contact us if you have any questions, our team is available and happy to help.
About Lipiodol
Lipiodol (Ethiodized Oil) Injection is a sterile injectable radio-opaque agent. Lipiodol is indicated for1:
- Selective hepatic intra-arterial use for imaging tumors in adults with known hepatocellular carcinoma (HCC) Learn more
- Lymphography in adults and pediatric patients Learn more
- Hysterosalpingography in adults Learn more
Lipiodol® is indicated for selective hepatic intra-arterial use for imaging tumors in adults with known HCC1
Lipiodol® can be used as an imaging agent during TACE in patients with known HCC.5
In HCC, Lipiodol can stay in the liver tumor for 4 weeks or longer facilitating re-imaging without re-administration.1
Following intra-arterial administration of Lipiodol, ethiodized oil retained in normal hepatic parenchyma is phagocytized by the Kupffer cells of the liver and washed out via the hepatic lymphatic system in about 2 to 4 weeks.1
Lipiodol can assist in the detection of daughter nodules in patients with known HCC.3
Primary Liver Cancer Epidemiology
Primary liver cancer and intrahepatic bile duct cancer in the United States (estimates, The American Cancer Society, 2023)4:
About 41,630 new cases (28,000 in men and 13,630 in women) will be diagnosed.
About 29,840 people (19,120 men and 10,720 women) will die of these cancers.
Liver cancer incidence rates have more than tripled since 1980, while the death rates have more than doubled during this time.
Lipiodol® is indicated for lymphography in adult and pediatric patients.1
Lymphangiography/Lymphography:
The use of imaging to visualize the lymphatic system following injection of a contrast agent:6
Lipiodol (ethiodized oil) is an oil-based radiopaque contrast agent indicated for lymphography adult and pediatric patients.1
Methods of Injection
Either Intranodal (A) or Transpedal (B) Lymphangiography7
Lipiodol® is the only oil based iodinated contrast agent indicated for HSG procedures.1
Hysterosalpingography is a radiological examination to evaluate the uterine cavity, Fallopian tubes, cervical canal & peritoneal cavity. It entails the injection of contrast medium and visualization under fluoroscopy. An HSG exam is indicated as part of an infertility work-up to diagnose blocked Fallopian tubes or uterine abnormalities. 2
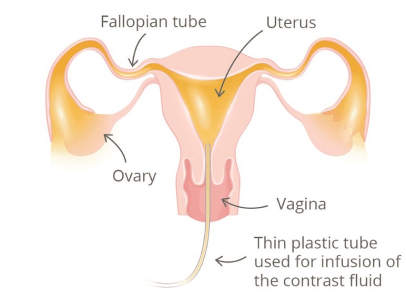
HSG Procedure 9
A woman is positioned under a fluoroscope (a x-ray imager that can take pictures during the study) on a table. The gynecologist or radiologist then examines the patient’s uterus and places a speculum in her vagina. Her cervix is cleaned, and a device (cannula) is placed into the opening of the cervix. The doctor gently fills the uterus with a liquid containing iodine (a fluid that can be seen by x-ray) through the cannula. The contrast will be seen as white on the image and can show the contour of the uterus as the liquid travels from the cannula, into the uterus, and through the fallopian tubes. As the contrast enters the tubes, it outlines the length of the tubes and spills out their ends if they are open. Abnormalities inside the uterine cavity may also be detected by the doctor observing the x-ray images when the fluid movement is disrupted by the abnormality. The HSG procedure is not designed to evaluate the ovaries or to diagnose endometriosis, nor can it identify fibroids that are outside of the endometrial cavity, either in the muscular part of the uterus, or on the outside of the uterus. Often, side views of the uterus and tubes are obtained by having the woman change her position on the table. After the HSG, a woman can immediately return to normal activities, although some doctors ask that she refrain from intercourse for a few days.
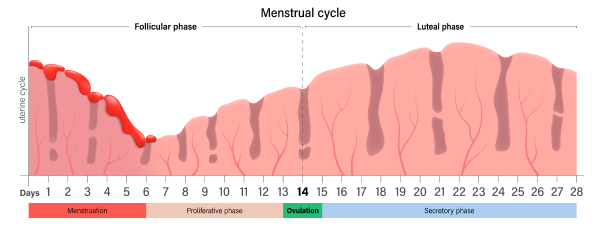
Characterization of HSG Findings 8 HSG is performed in the follicular phase of the menstrual cycle.
| Tubal abnormalities | Uterine cavity abnormalities |
|---|---|
|
|
When to Perform HSG Test 1
HSG is performed in the follicular phase of the menstrual cycle.
How does Lipiodol work in hepatic tumors?
Lipiodol opacifies vessels and body structures in the path of the flow of the contrast medium, permitting visualization of the internal structures.
In HCC, Lipiodol can stay in the tumor for 4 weeks or longer facilitating re-imaging without re-administration.1
Learn moreThe history of Lipiodol

Order Lipiodol & Qitexio
It’s easy to order. Contact us today.
Pulmonary and cerebral embolism can result from inadvertent intravascular injection or intravasation of Lipiodol. Inject Lipiodol slowly with radiologic monitoring; do not exceed recommended dose.
LIPIODOL® (ethiodized oil) injection is a prescription oil-based radio-opaque contrast agent indicated for:
- hysterosalpingography in adults
- lymphography in adult and pediatric patients
- selective hepatic intra-arterial use for imaging tumors in adults with known hepatocellular carcinoma (HCC)
LIPIODOL® is contraindicated in patients with hypersensitivity to LIPIODOL®, hyperthyroidism, traumatic injuries, recent hemorrhage or bleeding.
- LIPIODOL® Hysterosalpingography is contraindicated in pregnancy, acute pelvic inflammatory disease, marked cervical erosion, endocervicitis and intrauterine bleeding, in the immediate pre-or postmenstrual phase, and within 30 days of curettage or conization or patients with known or suspected reproductive tract neoplasia due to the risk of peritoneal spread of neoplasm.
- LIPIODOL® Lymphography is contraindicated in patients with a right to left cardiac shunt, advanced pulmonary disease, tissue trauma or hemorrhage, advanced neoplastic disease with expected lymphatic obstruction, previous surgery interrupting the lymphatic system, radiation therapy to the examined area.
- LIPIODOL® Selective Hepatic Intra-arterial Injection is contraindicated in the presence of dilated bile ducts unless external biliary drainage was performed before injection.
- Pulmonary and cerebral embolism may occur immediately or after a few hours to days from inadvertent systemic vascular injection or intravasation of LIPIODOL®. Avoid use in patients with severely impaired lung function, cardiorespiratory failure or right-sided cardiac overload.
- Anaphylactoid and anaphylactic reactions with cardiovascular, respiratory or cutaneous manifestations, ranging from mild to severe, including death, have uncommonly occurred following LIPIODOL® Avoid use in patients with a history of sensitivity to other iodinated contrast agents, bronchial asthma or allergic disorders because of an increased risk of a hypersensitivity reaction to LIPIODOL®.
- LIPIODOL® hepatic intra-arterial administration can exacerbate chronic liver disease.
- Iodinated contrast media can affect thyroid function because of the iodide content and can cause hyperthyroidism or hypothyroidism.
- Hysterosalpingography – Abdominal pain, foreign body reactions, exacerbation of pelvic inflammatory disease, salpingitis or pelvic peritonitis have been reported after the examination in case of latent infection.
- Lymphography – Lymphangitis, thrombophlebitis, edema or exacerbation of preexisting lymphedema, dyspnea and cough, iodism, allergic dermatitis, lipogranuloma, delayed healing at the site of incision.
- Selective Hepatic Intra-arterial Injection – Abdominal pain, nausea, and vomiting are the most common reactions; other reactions include hepatic vein thrombosis, hepatic ischemia, liver enzymes abnormalities, transitory decrease in liver function, liver decompensation and renal insufficiency. Procedural risks include vascular complications and infections.
- Pregnancy: The use of LIPIODOL® before or during pregnancy may interfere with thyroid function in both the pregnant woman and her fetus and may affect fetal development. Untreated hypothyroidism in pregnancy is associated with adverse perinatal outcomes, such as spontaneous abortion, preeclampsia, preterm birth, abruptio placentae, and fetal death. The use of LIPIODOL® before or during pregnancy causes iodide transfer across the placenta which may interfere with fetal thyroid function and may affect fetal development. Untreated hypothyroidism is also associated with increased fetal risk of low birth weight, fetal distress, and impaired neuropsychological development. Consider thyroid function testing during pregnancy if a woman was exposed to LIPIODOL® either before or during pregnancy, and also in infants whose mothers were exposed to LIPIODOL® before and during pregnancy or if clinically indicated.
- Pregnancy Testing: Confirm that the patient has a negative pregnancy test within 24 hours before LIPIODOL® administration for hysterosalpingography. Lactation: The use of LIPIODOL® may increase the concentration of iodide in human milk and may interfere with the thyroid function of the breastfed infant. Consider thyroid function testing in a breastfed infant whose mother was exposed to LIPIODOL® or if clinically indicated.
- Pediatric: For lymphography use a dose of minimum of 1 mL to a maximum of 6 mL according to the anatomical area to be visualized. Do not exceed 0.25 mL/kg. Administer the smallest possible amount of LIPIODOL® according to the anatomical area to be visualized.
- Geriatric: There are no studies conducted in geriatric patients.
- Renal Impairment: Prior to an intra-arterial administration of LIPIODOL® screen all patients for renal dysfunction by obtaining history and/or laboratory tests. Consider follow-up renal function assessments for patients with a history of renal dysfunction.
For more information, see Full Prescribing Information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
For further assistance, please contact LIPIODOL® Reimbursement Support at 1-855-368-2736, Monday–Friday, 7 am–7 pm ET.
Lipiodol® (Ethiodized Oil) Injection is a registered trademark of Guerbet LLC or its affiliates and is available by prescription only.
For more information, see Full Prescribing Information.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.